Is Dna Organic Or Inorganic

Inorganic chemical science deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as at that place is much overlap in the subdiscipline of organometallic chemistry. It has applications in every attribute of the chemic industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.[1]
Key concepts [edit]
Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding.[2] Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions Oii−. In any salt, the proportions of the ions are such that the electric charges abolish out, then that the bulk chemical compound is electrically neutral. The ions are described by their oxidation state and their ease of germination tin can be inferred from the ionization potential (for cations) or from the electron affinity (anions) of the parent elements.
The force of a bond in ionic compounds is known as lattice energy. It tin exist defined as the heat released when ions of opposite charge in the gas stage to combine into an ionic solid.[3] For instance, if we take a sodium and chlorine atom and combined them we would get , because this number is negative nosotros would accept an exothermic reaction, if this number was positive information technology would be an endothermic reaction. Another manner of describing lattice energy is the energy required to divide one mole of an ionic solid into a gas, this is the reverse of the previous description. It is not possible to determine these values experimentally due to the number of atmospheric condition that could influence the reaction but it can be estimated using the Born-Haber cycle
Important classes of inorganic compounds are the oxides, the carbonates, the sulfates, and the halides. Many inorganic compounds are characterized past high melting points. Inorganic salts typically are poor conductors in the solid state only rise slightly while molten.[iv] Other of import features include their high melting betoken and ease of crystallization. Where some salts (e.1000., NaCl) are very soluble in water, others (due east.yard., FeS) are not.
The simplest inorganic reaction is double deportation when in mixing of ii salts the ions are swapped without a change in oxidation land. In redox reactions one reactant, the oxidant, lowers its oxidation state and some other reactant, the reductant, has its oxidation state increased. The cyberspace event is an exchange of electrons. Electron exchange can occur indirectly too, e.1000., in batteries, a key concept in electrochemistry.
When i reactant contains hydrogen atoms, a reaction tin can take identify by exchanging protons in acid-base chemistry. In a more general definition, any chemical species capable of binding to electron pairs is chosen a Lewis acrid; conversely any molecule that tends to donate an electron pair is referred to as a Lewis base.[5] As a refinement of acid-base interactions, the HSAB theory takes into account polarizability and size of ions.
Inorganic compounds are found in nature as minerals.[6] Soil may contain fe sulfide as pyrite or calcium sulfate as gypsum.[seven] [8] Inorganic compounds are also establish multitasking as biomolecules: as electrolytes (sodium chloride), in free energy storage (ATP) or in construction (the polyphosphate backbone in Deoxyribonucleic acid).
The offset of import man-fabricated inorganic chemical compound was ammonium nitrate for soil fertilization through the Haber procedure.[nine] Inorganic compounds are synthesized for use as catalysts such as vanadium(V) oxide and titanium(Three) chloride, or as reagents in organic chemistry such as lithium aluminium hydride.
Subdivisions of inorganic chemistry are organometallic chemistry, cluster chemistry and bioinorganic chemistry. These fields are active areas of inquiry in inorganic chemistry, aimed toward new catalysts, superconductors, and therapies.
Industrial inorganic chemistry [edit]
Inorganic chemistry is a highly applied expanse of scientific discipline. Traditionally, the calibration of a nation's economic system could be evaluated by their productivity of sulfuric acid. The manufacturing of fertilizers, which often begins with the Haber-Bosch procedure, is another applied application of industrial inorganic chemistry.[10] [eleven]
Descriptive inorganic chemistry [edit]
Descriptive inorganic chemical science focuses on the nomenclature of compounds based on their backdrop. Partly the classification focuses on the position in the periodic table of the heaviest chemical element (the element with the highest atomic weight) in the compound, partly by grouping compounds by their structural similarities.
Coordination compounds [edit]
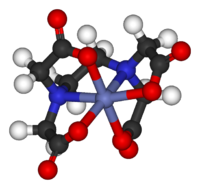
Classical coordination compounds feature metals bound to "lonely pairs" of electrons residing on the primary group atoms of ligands such every bit H2O, NH3, Cl−, and CN−. In modern coordination compounds almost all organic and inorganic compounds tin be used as ligands. The "metallic" usually is a metal from the groups three–thirteen, besides as the trans-lanthanides and trans-actinides, but from a certain perspective, all chemical compounds tin can be described as coordination complexes.
The stereochemistry of coordination complexes tin exist quite rich, every bit hinted at by Werner'south separation of two enantiomers of [Co((OH)2Co(NHiii)4)3]6+, an early on demonstration that chirality is not inherent to organic compounds. A topical theme within this specialization is supramolecular coordination chemistry.[12]
- Examples: [Co(EDTA)]−, [Co(NH3)6]three+, TiCliv(THF)2.
Master group compounds [edit]

These species feature elements from groups I, 2, 3, IV, V,VI, VII, 0 (excluding hydrogen) of the periodic table. Due to their often similar reactivity, the elements in group 3 (Sc, Y, and La) and grouping 12 (Zn, Cd, and Hg) are too mostly included, and the lanthanides and actinides are sometimes included also.[thirteen]
Master group compounds have been known since the beginnings of chemistry, e.g., elemental sulfur and the distillable white phosphorus. Experiments on oxygen, O2, by Lavoisier and Priestley not merely identified an of import diatomic gas, but opened the style for describing compounds and reactions co-ordinate to stoichiometric ratios. The discovery of a applied synthesis of ammonia using iron catalysts past Carl Bosch and Fritz Haber in the early 1900s securely impacted mankind, demonstrating the significance of inorganic chemical synthesis. Typical master group compounds are SiOtwo, SnCliv, and Northward2O. Many main grouping compounds can also be classed as "organometallic", every bit they incorporate organic groups, e.g., B(CH3)3). Master group compounds also occur in nature, e.g., phosphate in Deoxyribonucleic acid, and therefore may be classed as bioinorganic. Conversely, organic compounds lacking (many) hydrogen ligands can be classed equally "inorganic", such as the fullerenes, buckytubes and binary carbon oxides.
- Examples: tetrasulfur tetranitride S4Northwardiv, diborane B2Hhalf-dozen, silicones, buckminsterfullerene Cthreescore.
Element of group 0 compounds [edit]
Noble gases are elements which have filled valence electron shells in their neutral country, and are thus stable equally lone atoms. Historically known as being inert, methods were discovered to react with them. The trend within the grouping is for the larger elements to be more reactive. Xenon and krypton are more easily ionized, and can combine with extremely electronegative elements to make fluorides and oxides and class solid ionic compounds. Argon, neon, and helium are much less reactive, though in cosmochemistry ArH+ has been observed spectroscopically in interstellar gas. Noble gases can also be trapped in solids while not being directly coordinated in clathrates or in endohedral fullerenes.
- Examples: xenon hexafluoride XeFsix, xenon trioxide XeO3, krypton difluoride KrF2, argon fluorohydride HArF
Transition element compounds [edit]
Compounds containing metals from group 4 to 11 are considered transition metal compounds. Compounds with a metal from group 3 or 12 are sometimes as well incorporated into this group, but also oft classified as chief group compounds.
Transition metal compounds show a rich coordination chemistry, varying from tetrahedral for titanium (due east.g., TiCl4) to foursquare planar for some nickel complexes to octahedral for coordination complexes of cobalt. A range of transition metals tin can be found in biologically of import compounds, such as iron in hemoglobin.
- Examples: iron pentacarbonyl, titanium tetrachloride, cisplatin
Organometallic compounds [edit]

Ordinarily, organometallic compounds are considered to contain the Grand-C-H group.[14] The metal (M) in these species can either exist a main grouping element or a transition element. Operationally, the definition of an organometallic compound is more relaxed to include also highly lipophilic complexes such as metal carbonyls and even metal alkoxides.
Organometallic compounds are mainly considered a special category because organic ligands are often sensitive to hydrolysis or oxidation, necessitating that organometallic chemistry employs more specialized preparative methods than was traditional in Werner-blazon complexes. Synthetic methodology, especially the ability to manipulate complexes in solvents of low coordinating power, enabled the exploration of very weakly coordinating ligands such as hydrocarbons, H2, and N2. Because the ligands are petrochemicals in some sense, the area of organometallic chemistry has greatly benefited from its relevance to industry.
- Examples: Cyclopentadienyliron dicarbonyl dimer (C5Hfive)Fe(CO)2CHiii, ferrocene Fe(CvH5)2, molybdenum hexacarbonyl Mo(CO)vi, triethylborane EtthreeB, Tris(dibenzylideneacetone)dipalladium(0) Pdtwo(dba)3)
Cluster compounds [edit]
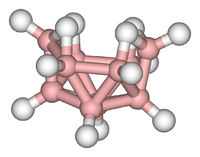

Clusters tin exist plant in all classes of chemical compounds. According to the unremarkably accepted definition, a cluster consists minimally of a triangular prepare of atoms that are straight bonded to each other. Simply metal-metal bonded dimetallic complexes are highly relevant to the surface area. Clusters occur in "pure" inorganic systems, organometallic chemistry, main group chemical science, and bioinorganic chemistry. The distinction between very big clusters and bulk solids is increasingly blurred. This interface is the chemical basis of nanoscience or nanotechnology and specifically arise from the study of breakthrough size furnishings in cadmium selenide clusters. Thus, large clusters can be described as an array of bound atoms intermediate in character between a molecule and a solid.
- Examples: Fe3(CO)12, BxHfourteen, [Mo6Clfourteen]2−, 4Fe-4S
Bioinorganic compounds [edit]

By definition, these compounds occur in nature, but the subfield includes anthropogenic species, such as pollutants (e.chiliad., methylmercury) and drugs (e.g., Cisplatin).[xv] The field, which incorporates many aspects of biochemistry, includes many kinds of compounds, east.g., the phosphates in Dna, and also metal complexes containing ligands that range from biological macromolecules, commonly peptides, to ill-defined species such as humic acid, and to water (e.1000., coordinated to gadolinium complexes employed for MRI). Traditionally bioinorganic chemistry focuses on electron- and energy-transfer in proteins relevant to respiration. Medicinal inorganic chemistry includes the report of both non-essential and essential elements with applications to diagnosis and therapies.
- Examples: hemoglobin, methylmercury, carboxypeptidase
Solid state compounds [edit]
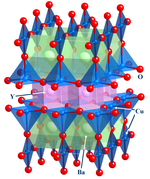
This of import expanse focuses on structure,[16] bonding, and the physical properties of materials. In practise, solid country inorganic chemistry uses techniques such equally crystallography to gain an understanding of the backdrop that result from collective interactions between the subunits of the solid. Included in solid state chemical science are metals and their alloys or intermetallic derivatives. Related fields are condensed matter physics, mineralogy, and materials scientific discipline.
- Examples: silicon chips, zeolites, YBa2Cu3O7
Theoretical inorganic chemical science [edit]
An alternative perspective on the expanse of inorganic chemical science begins with the Bohr model of the atom and, using the tools and models of theoretical chemistry and computational chemistry, expands into bonding in unproblematic and so more complicated molecules. Precise quantum mechanical descriptions for multielectron species, the province of inorganic chemistry, is difficult. This challenge has spawned many semi-quantitative or semi-empirical approaches including molecular orbital theory and ligand field theory, In parallel with these theoretical descriptions, judge methodologies are employed, including density functional theory.
Exceptions to theories, qualitative and quantitative, are extremely of import in the development of the field. For example, CuIi 2(OAc)iv(H2O)ii is almost diamagnetic beneath room temperature whereas crystal field theory predicts that the molecule would have 2 unpaired electrons. The disagreement betwixt qualitative theory (paramagnetic) and observation (diamagnetic) led to the development of models for magnetic coupling, such as the exchange interaction. These improved models led to the development of new magnetic materials and new technologies.
Qualitative theories [edit]

Inorganic chemistry has greatly benefited from qualitative theories. Such theories are easier to learn as they crave fiddling background in quantum theory. Within main group compounds, VSEPR theory powerfully predicts, or at least rationalizes, the structures of master grouping compounds, such equally an explanation for why NH3 is pyramidal whereas ClFiii is T-shaped. For the transition metals, crystal field theory allows one to empathize the magnetism of many simple complexes, such as why [Fe3(CN)6]three− has only i unpaired electron, whereas [FeIII(H2O)vi]3+ has five. A specially powerful qualitative arroyo to assessing the structure and reactivity begins with classifying molecules co-ordinate to electron counting, focusing on the numbers of valence electrons, ordinarily at the central atom in a molecule.
Molecular symmetry group theory [edit]
A central construct in inorganic chemistry is the theory of molecular symmetry.[17] Mathematical grouping theory provides the language to describe the shapes of molecules according to their betoken grouping symmetry. Grouping theory also enables factoring and simplification of theoretical calculations.
Spectroscopic features are analyzed and described with respect to the symmetry backdrop of the, inter alia, vibrational or electronic states. Knowledge of the symmetry properties of the ground and excited states allows 1 to predict the numbers and intensities of absorptions in vibrational and electronic spectra. A classic application of group theory is the prediction of the number of C-O vibrations in substituted metal carbonyl complexes. The most common applications of symmetry to spectroscopy involve vibrational and electronic spectra.
Group theory highlights commonalities and differences in the bonding of otherwise disparate species. For example, the metallic-based orbitals transform identically for WFvi and W(CO)6, merely the energies and populations of these orbitals differ significantly. A similar relationship exists COii and molecular beryllium difluoride.
Thermodynamics and inorganic chemistry [edit]
An alternative quantitative approach to inorganic chemical science focuses on energies of reactions. This approach is highly traditional and empirical, simply it is as well useful. Broad concepts that are couched in thermodynamic terms include redox potential, acidity, phase changes. A classic concept in inorganic thermodynamics is the Built-in-Haber bike, which is used for assessing the energies of elementary processes such equally electron affinity, some of which cannot exist observed directly.
Mechanistic inorganic chemistry [edit]
An important aspect of inorganic chemical science focuses on reaction pathways, i.e. reaction mechanisms.
Master group elements and lanthanides [edit]
The mechanisms of main grouping compounds of groups thirteen-xviii are usually discussed in the context of organic chemistry (organic compounds are main grouping compounds, subsequently all). Elements heavier than C, N, O, and F frequently form compounds with more electrons than predicted by the octet dominion, as explained in the article on hypervalent molecules. The mechanisms of their reactions differ from organic compounds for this reason. Elements lighter than carbon (B, Be, Li) as well as Al and Mg often grade electron-deficient structures that are electronically akin to carbocations. Such electron-deficient species tend to react via associative pathways. The chemical science of the lanthanides mirrors many aspects of chemistry seen for aluminium.
Transition metal complexes [edit]
Transition metallic and main group compounds often react differently.[18] The of import office of d-orbitals in bonding strongly influences the pathways and rates of ligand substitution and dissociation. These themes are covered in articles on coordination chemistry and ligand. Both associative and dissociative pathways are observed.
An overarching aspect of mechanistic transition metallic chemistry is the kinetic lability of the complex illustrated by the exchange of free and spring h2o in the prototypical complexes [Yard(H2O)half-dozen]n+:
- [1000(H2O)6]n+ + 6 H2O* → [M(HtwoO*)6]due north+ + 6 H2O
- where H2O* denotes isotopically enriched water, e.g., Hii 17O
The rates of water exchange varies past 20 orders of magnitude across the periodic tabular array, with lanthanide complexes at 1 extreme and Ir(Iii) species being the slowest.
Redox reactions [edit]
Redox reactions are prevalent for the transition elements. Two classes of redox reaction are considered: atom-transfer reactions, such as oxidative addition/reductive elimination, and electron-transfer. A fundamental redox reaction is "cocky-commutation", which involves the degenerate reaction between an oxidant and a reductant. For example, permanganate and its one-electron reduced relative manganate exchange one electron:
- [MnOfour]− + [Mn*O4]ii− → [MnO4]two− + [Mn*O4]−
Reactions at ligands [edit]
Coordinated ligands brandish reactivity distinct from the gratuitous ligands. For case, the acerbity of the ammonia ligands in [Co(NHthree)6]3+ is elevated relative to NHiii itself. Alkenes bound to metallic cations are reactive toward nucleophiles whereas alkenes usually are not. The big and industrially important expanse of catalysis hinges on the ability of metals to modify the reactivity of organic ligands. Homogeneous catalysis occurs in solution and heterogeneous catalysis occurs when gaseous or dissolved substrates collaborate with surfaces of solids. Traditionally homogeneous catalysis is considered part of organometallic chemistry and heterogeneous catalysis is discussed in the context of surface science, a subfield of solid state chemical science. But the basic inorganic chemical principles are the aforementioned. Transition metals, well-nigh uniquely, react with small molecules such as CO, H2, O2, and C2H4. The industrial significance of these feedstocks drives the agile area of catalysis. Ligands can likewise undergo ligand transfer reactions such every bit transmetalation.
Label of inorganic compounds [edit]
Because of the various range of elements and the correspondingly diverse backdrop of the resulting derivatives, inorganic chemistry is closely associated with many methods of analysis. Older methods tended to examine bulk properties such as the conductivity of solutions, melting points, solubility, and acidity. With the appearance of quantum theory and the corresponding expansion of electronic apparatus, new tools have been introduced to probe the electronic properties of inorganic molecules and solids. Often these measurements provide insights relevant to theoretical models. Unremarkably encountered techniques are:
- 10-ray crystallography: This technique allows for the 3D determination of molecular structures.
- Dual polarisation interferometer: This technique measures the conformation and conformational change of molecules.
- Various forms of spectroscopy:
- Ultraviolet-visible spectroscopy: Historically, this has been an of import tool, since many inorganic compounds are strongly colored
- NMR spectroscopy: Besides iH and 13C many other NMR-active nuclei (e.g., xiB, 19F, 31P, and 195Pt) can requite important information on compound properties and structure. The NMR of paramagnetic species can provide important structural information. Proton (1H) NMR is besides important because the calorie-free hydrogen nucleus is non easily detected by X-ray crystallography.
- Infrared spectroscopy: Mostly for absorptions from carbonyl ligands
- Electron nuclear double resonance (ENDOR) spectroscopy
- Mössbauer spectroscopy
- Electron-spin resonance: ESR (or EPR) allows for the measurement of the surroundings of paramagnetic metal centres.
- Electrochemistry: Cyclic voltammetry and related techniques probe the redox characteristics of compounds.
Synthetic inorganic chemistry [edit]
Although some inorganic species can be obtained in pure form from nature, most are synthesized in chemical plants and in the laboratory.
Inorganic synthetic methods tin can be classified roughly according to the volatility or solubility of the component reactants.[nineteen] Soluble inorganic compounds are prepared using methods of organic synthesis. For metal-containing compounds that are reactive toward air, Schlenk line and glove box techniques are followed. Volatile compounds and gases are manipulated in "vacuum manifolds" consisting of glass pipage interconnected through valves, the entirety of which can be evacuated to 0.001 mm Hg or less. Compounds are condensed using liquid nitrogen (b.p. 78K) or other cryogens. Solids are typically prepared using tube furnaces, the reactants and products being sealed in containers, ofttimes made of fused silica (amorphous SiO2) but sometimes more specialized materials such every bit welded Ta tubes or Pt "boats". Products and reactants are transported between temperature zones to bulldoze reactions.
See also [edit]
- Important publications in inorganic chemistry
References [edit]
- ^ "Careers in Chemistry: Inorganic Chemical science". American Chemic Social club. Archived from the original on 2012-10-29.
- ^ Coll, Richard G.; Treagust, David F. (2003). "Investigation of secondary schoolhouse, undergraduate, and graduate learners' mental models of ionic bonding". Journal of Enquiry in Science Didactics. forty (5): 464–486. Bibcode:2003JRScT..40..464C. doi:10.1002/tea.10085. ISSN 0022-4308.
- ^ al., Brownish, Theodore E., LeMay, Eugene H., Bursten, Bruce E., et (2012). Chemical science: The Primal Science. ISBN978-0-321-74105-9. OCLC 911672522.
- ^ Chliatzou, Ch. D.; Assael, M. J.; Antoniadis, K. D.; Huber, Thousand. L.; Wakeham, West. A. (2018). "Reference Correlations for the Thermal Conductivity of 13 Inorganic Molten Salts". Journal of Physical and Chemical Reference Data. 47 (3): 033104. Bibcode:2018JPCRD..47c3104C. doi:10.1063/i.5052343. ISSN 0047-2689. PMC6459620. PMID 30983644.
- ^ Jensen, William B. (1978). "The Lewis acrid-base definitions: a condition report". Chemical Reviews. 78 (1): 1–22. doi:10.1021/cr60311a002. ISSN 0009-2665.
- ^ Burns, P. C. (2005-12-01). "U6+ Minerals and Inorganic Compounds: Insights into an Expanded Structural Hierarchy of Crystal Structures". The Canadian Mineralogist. 43 (6): 1839–1894. doi:10.2113/gscanmin.43.6.1839. ISSN 0008-4476.
- ^ Shainberg, I.; Sumner, Chiliad. E.; Miller, Westward. P.; Farina, M. P. W.; Pavan, Yard. A.; Fey, M. V. (1989), Lal, Rattan; Stewart, B. A. (eds.), "Employ of Gypsum on Soils: A Review", Soil Restoration, New York, NY: Springer New York, vol. 17, pp. 1–111, doi:x.1007/978-1-4612-3532-3_1, ISBN978-i-4612-7684-5 , retrieved 2022-08-21
- ^
- ^ Witschi, H. (2000-05-01). "Fritz Haber: 1868-1934". Toxicological Sciences. 55 (1): 1–2. doi:10.1093/toxsci/55.i.1. PMID 10788553.
- ^ Guo, Jianping; Chen, Ping (2021). "Ammonia history in the making". Nature Catalysis. iv (9): 734–735. doi:10.1038/s41929-021-00676-0. ISSN 2520-1158. S2CID 237588318.
- ^ Leigh, K. J. (2004), Smith, Barry E.; Richards, Raymond L.; Newton, William E. (eds.), "Haber-Bosch and Other Industrial Processes", Catalysts for Nitrogen Fixation, Dordrecht: Springer Netherlands, pp. 33–54, doi:10.1007/978-1-4020-3611-8_2, ISBN978-90-481-6675-vi , retrieved 2022-08-21
- ^ Lehn, J.One thousand. (1995). Supramolecular Chemistry: Concepts and Perspectives. Weinheim: VCH. ISBN978-three-527-29311-7.
- ^ Greenwood, Norman Due north.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^ Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Curtailed Introduction (second ed.). Weinheim: Wiley-VCH. ISBN978-3-527-28164-0.
- ^ S.J. Lippard; J.M. Berg (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: University Scientific discipline Books. ISBN978-0-935702-73-six.
- ^ Wells, A.F. (1984). Structural Inorganic Chemical science. Oxford: Clarendon Printing.
- ^ Cotton, F.A. (1990). Chemical Applications of Group Theory (tertiary ed.). New York: John Wiley & Sons. ISBN978-0-471-51094-9.
- ^ R.G. Wilkins (1991). Kinetics and Mechanism of Reactions of Transition metal Complexes (second ed.). Wiley-VCH. ISBN978-3-527-28389-vii.
- ^ Girolami, G.S.; Rauchfuss, T.B.; Angelici, R.J. (1999). Synthesis and Technique in Inorganic Chemistry (3rd ed.). Mill Valley, CA: University Science Books. ISBN978-0-935702-48-4.
Is Dna Organic Or Inorganic,
Source: https://en.wikipedia.org/wiki/Inorganic_chemistry
Posted by: johnsoncrivair.blogspot.com




0 Response to "Is Dna Organic Or Inorganic"
Post a Comment